Three faculty members from the Auburn University College of...


Three faculty members from the Auburn University College of...
“Human” episode airing Saturday to feature Penick’s research on urban ants An episode of Planet Earth III airing in the U.S. this Saturday, Dec. 16, was made possible with the help of Assistant Professor Clint Penick in the Department of Entomology and Plant...
Farmers have a hard enough time battling the pests they can see. The ones they can’t see — like plant parasitic nematodes — present another challenge altogether. These worm-like, sometimes microscopic animals are major agricultural pathogens that attack Alabama crop...

The USDA National Institute of Food and Agriculture recently awarded a $3.9 million, five-year grant to a team of researchers to enhance honey bee health for...
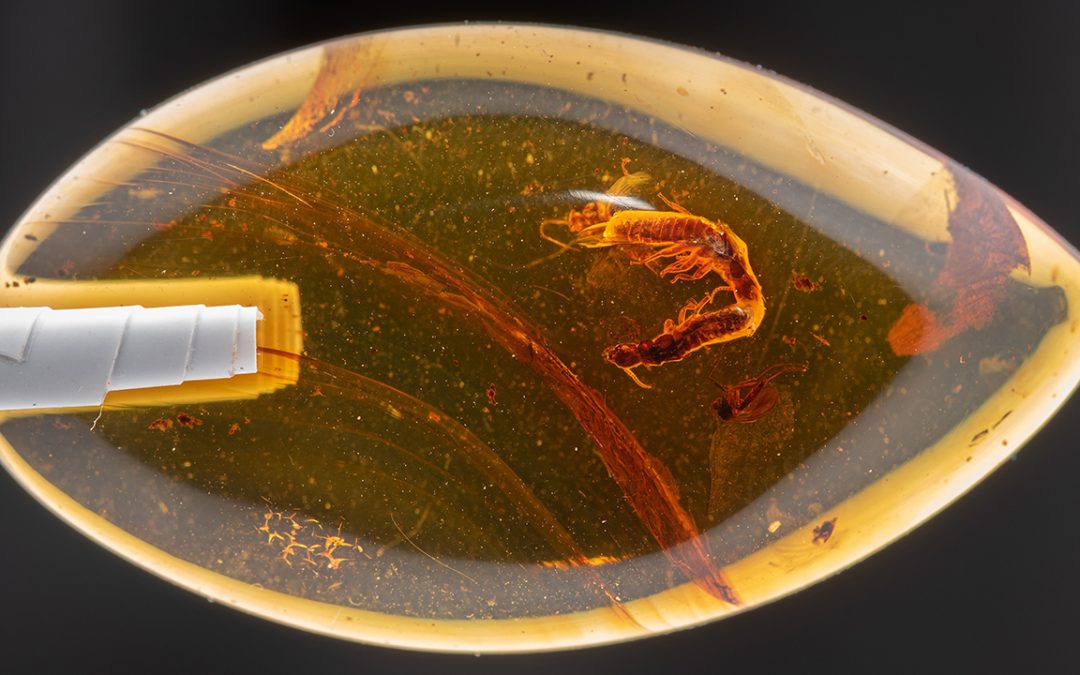
Mizumoto recreates fossilization process to test tandem run hypothesis An assistant professor in the Auburn University Department of Entomology & Plant Pathology...

An endowed professor in the Auburn University College of Agriculture is listed as among the world’s top 2% of entomologists in a database announced recently by Stanford...

De La Fuente first Auburn researcher to receive EU-funded award An Auburn University researcher and professor is part of an international team of scientists who are playing a pivotal role in combating a disease that is threatening major tree crops around the world....

Auburn agriculture senior finds success in his major Do you know what applied biotechnology is? Harrison Estes — a senior majoring in applied biotechnology — defines it as “the study and practice of genetic modification for industrial, medical and agricultural...

Alabama Watershed Stewards and Alabama Water Watch, along with the AU Bees Lab and Westervelt Ecological Resources, are partnering with the city of Auburn through a recently awarded grant from the National Fish and Wildlife Foundation for 2022-23. Alabama Watershed...

One College of Agriculture faculty member was recently reappointed an eminent scholar and two others received endowed professorships. Rex Dunham, alumni professor in the School of Fisheries, Aquaculture and Aquatic Sciences, was reappointed the Butler-Cunningham...

Few students have been as actively involved in the Department of Entomology and Plant Pathology at Auburn University as Seun Oladipupo, a Ph.D. candidate who plans to complete his degree in May. According to Entomology and Plant Pathology Chair David Held, Oladipupo...

By Mike Jernigan So, imagine you’re a female mosquito. You’re patiently circling, waiting to pounce, while your unsuspecting victim enjoys a picnic with no idea they have unknowingly been placed on your menu. Your multi-lensed eyes have confirmed what your carbon...

Local Auburn business the Collegiate Hotel recently announced the creation of The Brian E. and Kimberly A. Wirth Family Annual Fund for Excellence, also known as the Bee Excellent Fund, supporting the AU-Bees Lab in the College of Agriculture at Auburn University. ...

From University of Maryland Beekeepers across the United States lost 45.5% of their managed honey bee colonies from April 2020 to April 2021, according to preliminary results of the 15th annual nationwide survey conducted by the nonprofit Bee Informed Partnership...

By Jacqueline Kochak More than 80 percent of Americans live in expanding urban areas, and suburbanites are still craving greenspace as farmland gives way to housing developments and big-box stores. The problem is that proud homeowners use significantly more...

Students in three College of Agriculture departments performed well in regional, national and international competitions this spring. A team from the Department of Horticulture placed first in its size group (10-24 students) in the 45th National Collegiate Landscape...

From exploring the viability of new crops such as hemp and grapes to protecting traditional crops such as peanuts and cotton, the College of Agriculture’s Production Agriculture Research, or PAR grants program, is working to provide immediate solutions...

By Jacqueline Kochak An Auburn University researcher has joined with European scientists in an attempt to decipher the disease process caused by one of the world’s most harmful plant pathogens, Xylella fastidiosa. The bacterium’s impact has been nothing short of...

The Departmental Entomology Team comprising students in the College of Agriculture placed first in the 2020 Entomology Games held on Nov. 16 by the Entomology Society of America. The Auburn entomology team includes team captain Seun Olaitan Oladipupo, Madison...
David Held has been named chair of Auburn University’s Department of Entomology and Plant Pathology, effective October 1. Held has been a faculty member in the department since 2008. “I believe Dr. Held’s extensive experience in research, teaching and extension have...

Two Auburn University College of Agriculture researchers received the Foundation for Food and Agriculture Research (FFAR) 2019 New Innovator Award.

Elina Coneva was appointed to the William A. Jr. and Cecelia Dozier Endowed Professorship and Kathy Lawrence to the Joseph Kloepper Professorship.

Auburn Entomologist John Beckmann was awarded $868,145 to develop a lightweight material that blocks mosquito bites and retains coolness in hot weather.

Neha Potnis, assistant professor in the College of Agriculture’s Department of Entomology and Plant Pathology, and Matt Waters, assistant professor in the Department of Crop, Soil and Environmental Sciences, have been named NSF Early Career award winners.

Dunham, Appel tapped for endowed professor positions.

A recent doctoral graduate in the College of Agriculture’s entomology program has completed the first scientific classification and identification study of a group of insects, phylloxerans – an insect similar to an aphid – that has been undertaken in more than a century.

The Auburn University College of Agriculture’s E.T. York Distinguished Lecturer Series will present U.S. Department of Agriculture Deputy Undersecretary Scott Hutchins as the Fall 2019 York Lecturer Thursday, Sept. 26, in Auburn. In the lecture, set for 4 p.m. in the...

De La Fuente first Auburn researcher to receive EU-funded award An Auburn University researcher and professor is part of an international team of scientists who are playing a pivotal role in combating a disease that is threatening major tree crops around the world....

Auburn agriculture senior finds success in his major Do you know what applied biotechnology is? Harrison Estes — a senior majoring in applied biotechnology — defines it as “the study and practice of genetic modification for industrial, medical and agricultural...

Alabama Watershed Stewards and Alabama Water Watch, along with the AU Bees Lab and Westervelt Ecological Resources, are partnering with the city of Auburn through a recently awarded grant from the National Fish and Wildlife Foundation for 2022-23. Alabama Watershed...

One College of Agriculture faculty member was recently reappointed an eminent scholar and two others received endowed professorships. Rex Dunham, alumni professor in the School of Fisheries, Aquaculture and Aquatic Sciences, was reappointed the Butler-Cunningham...

Few students have been as actively involved in the Department of Entomology and Plant Pathology at Auburn University as Seun Oladipupo, a Ph.D. candidate who plans to complete his degree in May. According to Entomology and Plant Pathology Chair David Held, Oladipupo...

By Mike Jernigan So, imagine you’re a female mosquito. You’re patiently circling, waiting to pounce, while your unsuspecting victim enjoys a picnic with no idea they have unknowingly been placed on your menu. Your multi-lensed eyes have confirmed what your carbon...

Local Auburn business the Collegiate Hotel recently announced the creation of The Brian E. and Kimberly A. Wirth Family Annual Fund for Excellence, also known as the Bee Excellent Fund, supporting the AU-Bees Lab in the College of Agriculture at Auburn University. ...

From University of Maryland Beekeepers across the United States lost 45.5% of their managed honey bee colonies from April 2020 to April 2021, according to preliminary results of the 15th annual nationwide survey conducted by the nonprofit Bee Informed Partnership...

By Jacqueline Kochak More than 80 percent of Americans live in expanding urban areas, and suburbanites are still craving greenspace as farmland gives way to housing developments and big-box stores. The problem is that proud homeowners use significantly more...

Students in three College of Agriculture departments performed well in regional, national and international competitions this spring. A team from the Department of Horticulture placed first in its size group (10-24 students) in the 45th National Collegiate Landscape...

From exploring the viability of new crops such as hemp and grapes to protecting traditional crops such as peanuts and cotton, the College of Agriculture’s Production Agriculture Research, or PAR grants program, is working to provide immediate solutions...

By Jacqueline Kochak An Auburn University researcher has joined with European scientists in an attempt to decipher the disease process caused by one of the world’s most harmful plant pathogens, Xylella fastidiosa. The bacterium’s impact has been nothing short of...

The Departmental Entomology Team comprising students in the College of Agriculture placed first in the 2020 Entomology Games held on Nov. 16 by the Entomology Society of America. The Auburn entomology team includes team captain Seun Olaitan Oladipupo, Madison...
David Held has been named chair of Auburn University’s Department of Entomology and Plant Pathology, effective October 1. Held has been a faculty member in the department since 2008. “I believe Dr. Held’s extensive experience in research, teaching and extension have...

Two Auburn University College of Agriculture researchers received the Foundation for Food and Agriculture Research (FFAR) 2019 New Innovator Award.

Elina Coneva was appointed to the William A. Jr. and Cecelia Dozier Endowed Professorship and Kathy Lawrence to the Joseph Kloepper Professorship.

Auburn Entomologist John Beckmann was awarded $868,145 to develop a lightweight material that blocks mosquito bites and retains coolness in hot weather.

Neha Potnis, assistant professor in the College of Agriculture’s Department of Entomology and Plant Pathology, and Matt Waters, assistant professor in the Department of Crop, Soil and Environmental Sciences, have been named NSF Early Career award winners.

Dunham, Appel tapped for endowed professor positions.

A recent doctoral graduate in the College of Agriculture’s entomology program has completed the first scientific classification and identification study of a group of insects, phylloxerans – an insect similar to an aphid – that has been undertaken in more than a century.

The Auburn University College of Agriculture’s E.T. York Distinguished Lecturer Series will present U.S. Department of Agriculture Deputy Undersecretary Scott Hutchins as the Fall 2019 York Lecturer Thursday, Sept. 26, in Auburn. In the lecture, set for 4 p.m. in the...